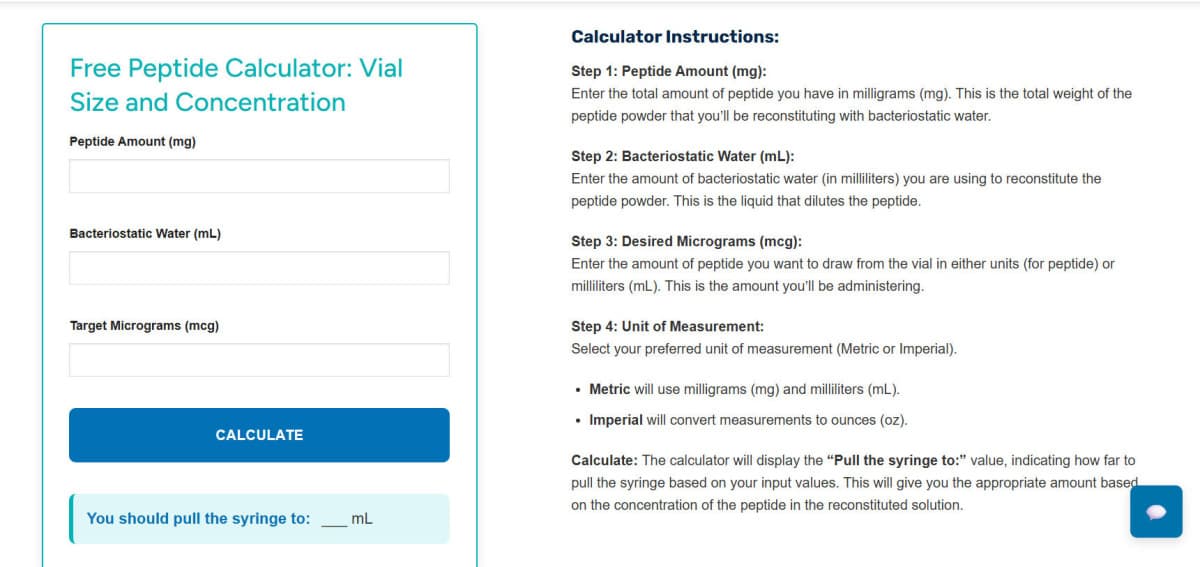
Accurate peptide preparation and reconstitution techniques are fundamental to maintaining research integrity and experimental reproducibility across scientific disciplines. The precise calculation of peptide concentrations forms the foundation of reliable research workflows, where concentration is determined by dividing peptide mass by diluent volume. Understanding unit conversions becomes critical when working with different measurement systems, particularly when converting between milligrams, micrograms, and molar concentrations based on molecular weight.
The reconstitution process requires careful attention to sterile techniques and appropriate solvent selection. Researchers must choose from various diluent options including bacteriostatic water for multi-use vials, sterile water for single-use aliquots, DMSO for hydrophobic peptides, or low percent acid for charged peptides. The step-by-step procedure involves setting up a clean workspace, disinfecting vial septums, slowly injecting calculated diluent volumes along vial walls, and gentle swirling until complete dissolution occurs without vigorous vortexing that could cause aggregation.
Proper storage conditions significantly impact peptide stability and experimental outcomes. Lyophilized peptides require cold, dry environments at -20°C for short-term storage or -80°C for long-term preservation, protected from light and moisture. Reconstituted peptides need refrigeration for immediate use or freezing at -20°C or -80°C for extended storage, with careful attention to minimizing freeze-thaw cycles. Researchers can find additional technical resources and guidance at https://lotilabs.com to support their laboratory practices.
Troubleshooting solubility and aggregation issues represents a common challenge in peptide research. When facing incomplete dissolution or precipitation, researchers should begin with gentle swirling and equilibration time, progress to brief sonication if necessary, and cautiously add small amounts of DMSO or low percent acid for stubborn peptides. Preventive strategies include proper solvent selection, slow addition to buffers, maintaining appropriate pH and ionic strength, and aliquoting to reduce freeze-thaw cycles.
Documentation and labeling practices ensure research transparency and reproducibility. Every step including solvent choices, concentration calculations, and any adjustments must be thoroughly recorded. All aliquots require clear labeling with concentration, solvent type, preparation date, and any modifications. These meticulous practices combined with sterile handling techniques and appropriate syringe selection for small-volume measurements contribute significantly to reducing experimental error and maintaining research quality across multiple studies and laboratories.